Often, a colony does not die just due to one of these factors. It’s the additive and synergistic effects that work together to gradually weaken, and ultimately result in the failure of the colony. An issue coming to the attention to more and more researchers is the sub-lethal impacts of pesticides. Even when pesticides are not lethal to honey bees, they can influence them physiologically and behaviorally, sometimes at very low doses.
When I decided to take on a 3-semester long research thesis, I knew I wanted to look at sub-lethal impacts of pesticides on honey bees. I chose a very specific type of behavioral impact that many pesticides are known to influence – the learning ability of honey bees.
 A cage of bees, complete with all feeders and treatment
A cage of bees, complete with all feeders and treatment After reading up on past studies looking at pesticide effects on learning ability in honey bees, I found the pesticide I wanted to investigate – fluvalinate. Fluvalinate is a pesticide that’s actually used to promote honey bee health, by eliminating the parasitic mite Varroa destructor that’s considered one of the largest drivers of bee decline today. Fluvalinate comes in a plastic strip – which is actually only comprised of 10% of the active ingredient and 90% other materials to stabilize it into a strip. The strip is left in a colony for 6-8 weeks, and reduces mite populations. This pesticide in particular leaves a lot of residues in the wax comb of colonies. According to the National Honey Bee Survey 94.8% of wax samples (n=194) were contaminated with fluvalinate. Some beekeepers have even reported that there are fluvalinate residues in their comb even though they have never even used the miticide. In past research, fluvalinate did show an impact on honey bee learning after exposure.
Past research involved collecting foragers flying out of an established colony, exposing them to fluvalinate, and testing how well they could learn associations a few hours later. But wait – I thought – if treatments are administered long-term directly inside a colony, are bees of all ages exposed? And are they exposed over a very long period of time? These were the questions that drove me to investigate the hypotheses that I did.
My past experience working with Dr. Samuel Ramsay taught me all about running cage studies – the practice of raising small populations of bees in cages inside an incubator. I decided to merge this experience and the questions I wanted to answer. Using a cage-study design would allow me to control the age of the bees I was exposing, and basically just administer a mini, scaled-down dosage of fluvalinate, almost exactly the way they would experience it in a colony.
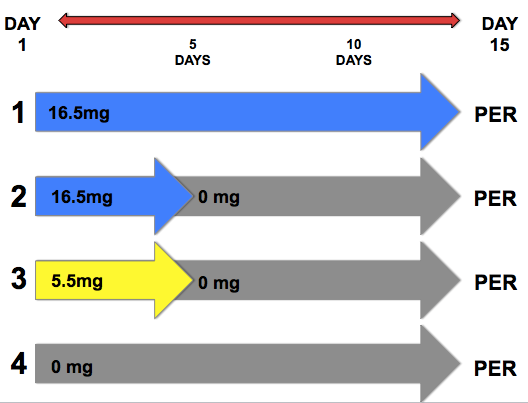
Using the manufacturer-prescribed dosage, the volume of a normal honey bee colony, and the volume of the type of cage I would use, I found a standard, realistic dose based upon habitat volume to use for my caged bees. I would expose groups of bees to this dose for either 0, 5, or 15 days. I also picked a small dose – just less than half of the standard – so I could compare the effects of dosage on the cages. This small dose I only gave to one treatment group for a length of 5 days.
 A harnessed bee, ready to be trained using PER!
A harnessed bee, ready to be trained using PER! The fun part (and by fun, I mean stressful and time-consuming, yet enjoyable) of each run was training day. Training day occurred on day 15 of the experiment, on which a lucky few bees would be plucked from each cup and I would attempt to train them, in order to measure the group’s learning ability.
To measure learning ability, I used an already-standardized method that’s been used since the 1950's. It’s called the Proboscis Extension Response, or PER, and it takes advantage of a bee’s natural reflex to extend its proboscis when confronted with sugar water in order to condition the bee.
Essentially, the method involves exposing a bee to some kind of unrelated stimulus, which is usually a floral odor. The bee is not familiar with this scent, and therefore has no inherent reaction to it. Then, the trainer will touch a drop of sugar water to the bee’s antennae, prompting it to extend its proboscis. The bee is then fed this sugar water to “reward” it for extending its proboscis.
Every training day, I aimed to attempt PER on 5 bees from every cup, which was 10 bees from every treatment group. Of course, I could not always train exactly 10 because there are very strict criteria upon which I would have to eliminate bees from training. If the bee ever did not respond to the sugar water, in any acquisition trial, I eliminated it from the group. This could happen if the bee was stressed, tired, or just not hungry enough to want to consume the sugar. When working with living creatures, you often abide by their rules, not your own, and things do not always go as planned.
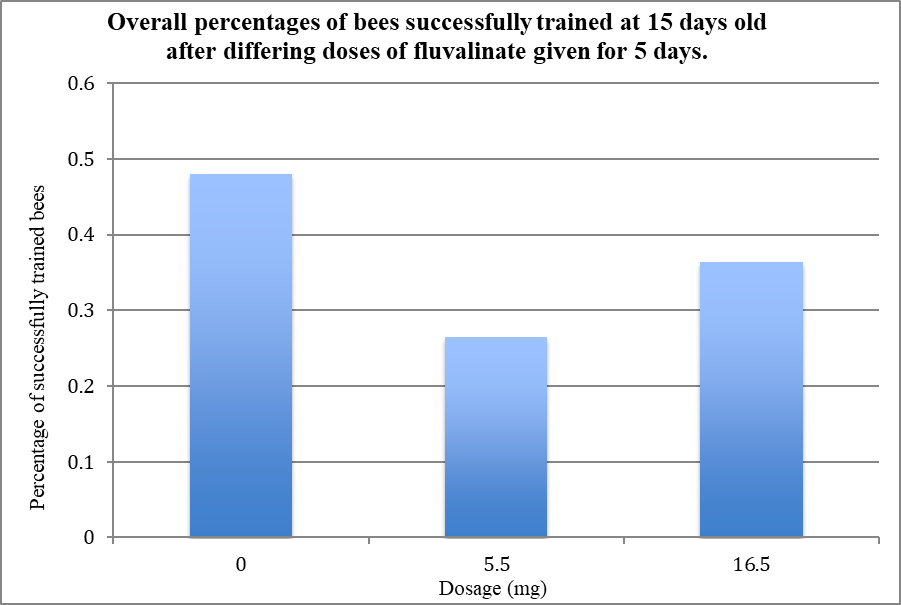
Despite attempting to train less bees than I had hoped, I still obtained a substantial amount of data. I analyzed the data for significance, and got some interesting results!
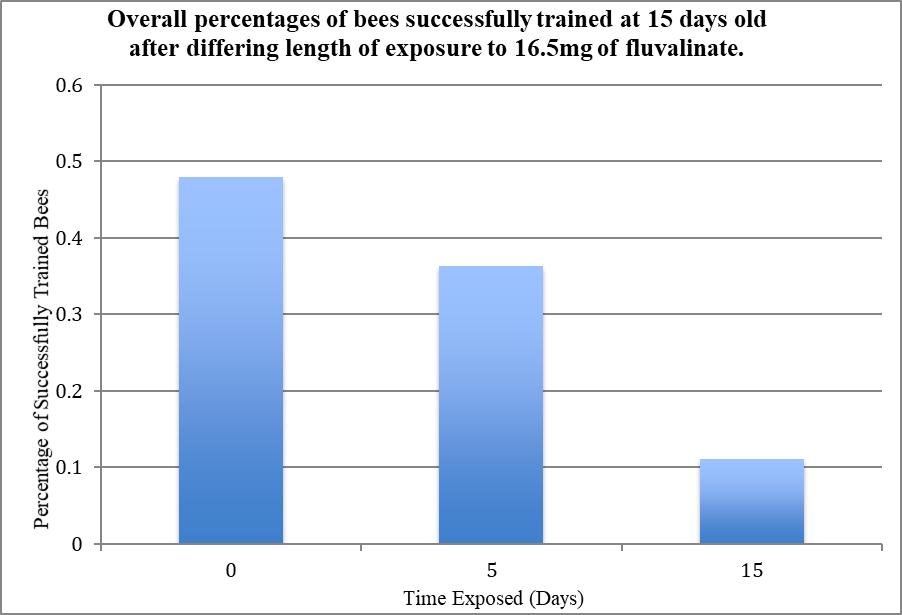
The surprising data from the first graph makes more sense in light of this. In the groups where I compared dosage, I only used an exposure period of 5 days. The second graph proves that 5 days is not long enough for the fluvalinate to really affect the rate of the bees’ learning! So I could not have expected to see significant results here.
The results from the experiment conducted here imply that fluvalinate, the pesticide we often use to keep bees healthy, could just be harming them in a different way. It would be very hard to tell that a colony died because its bees were not able to learn, and it is very unlikely that this factor alone would be what killed them. The more likely scenario is that less efficient foraging and communication, as a result from a reduction in learning would weakening the bees in conjunction with other impacts like weakened immunity and pathogens from varroa mites, poor nutrition due to a lack of diverse food sources, or severe weather fluctuations.
This warrants further thinking into how we treat our honey bees. We have to think about the trade-offs of varroa mite control vs. accidentally poisoning the colony. Not to say treatments for varroa should stop, but perhaps more research on the effects of miticides on the bees themselves should be conducted, and a product with the lowest ratio of harmful effects on bees to effective control of varroa can be promoted for use among beekeepers.
Author's note: I received High Honors in Entomology for my thesis defense!
AuthorMeg Wickless recently graduated with a B.S. in Biological Sciences. She has been working with the vanEngelsdorp lab since January 2016, and will continue working with us post-graduation. Meg is largely involved with honey bee field work, ongoing graduate research, and day-to-day lab operations. She hopes to attend graduate school in the future to further study ecology and the environment. |


 RSS Feed
RSS Feed